Background Unplanned 30-day readmissions are a frequent complication of acute myeloid leukemia (AML) therapy, with rates of 30-60% from published studies, including a 30% readmission rate (RaR) for induction and 40-50% RaR during consolidation. However, the data is still limited regarding predictors for readmissions.
Methods We retrospectively reviewed hospitalizations for AML for adult patients >/= 18 years at our institution between January 2018 and December 2018. Unplanned readmissions were defined as any hospitalization within 30-days after discharge from the index admission, excluding all elective re-admissions for scheduled chemotherapy. Patients >/= 60 years of age receiving their initial induction therapy remained hospitalized for at least 21 days until bone marrow analysis and neutrophil count above 500/mm3.
The primary objective was to identify the 30-day unplanned readmission rates, cause of readmissions, and predicted factors for readmissions. The secondary outcome included 30-day readmission mortality rate and inpatient mortality rate.
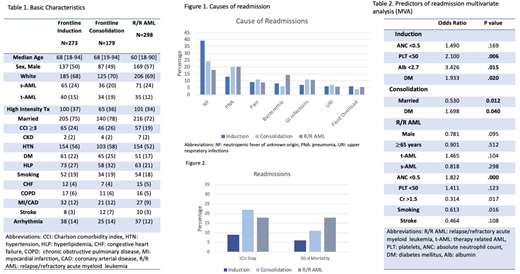
Results There were a total of 1681 AML hospitalizations, 393 admissions for 273 pts (average 1.44 re-admit/pt) during frontline induction, 364 for 179 pts (average 2.03) during consolidation and 924 for 298 pts (average 3.1) with relapsed/refractory AML (R/R AML). The median age was 68 years for the frontline induction and consolidation groups and 60 years for R/R AML patients. Baseline characteristics are listed in Table 1. The median length of index admission was 21 days for induction, 4 days during consolidation, and 7 days for R/R AML. The 30-day unplanned RaR was 27% for induction, 30% for consolidation, and 46% for R/R AML, with a median time to readmission of 7 days, 11 days, and 7 days, respectively.
The most common causes of readmissions were infections (80%, 75% and 75% during induction, consolidation and R/R AML respectively), including 39% neutropenic fever of unknown origin (NF), 13% pneumonia, and 8% bacteremia for induction; 24% NF, 20% pneumonia and 11% gastrointestinal infections during consolidation; and 18% NF, 20% pneumonia and 14% bacteremia for R/R AML. (Figure 1)
The median length of readmission was 6 days for frontline pts and 8 days for R/R AML.
The rate of ICU stay was 9% for induction, and 22% for consolidation, and 18% for R/R AML. (Figure 2). There was a significant increase of ICU stay for readmissions during consolidation (22% vs 5%, p=0.010) and R/R AML (18% vs 10% p=<0.001 for R/R AML) compared to non-readmissions.
We confirmed hypoalbuminemia (OR 3.42; p= 0.006), thrombocytopenia (OR 2.10; p= 0.015) and history of diabetes (OR 1.93; p= 0.020) as an independent predictor for readmissions during induction by multivariate analysis, while history of diabetes was significantly associated with increased risk of readmission for consolidation (OR 1.69; p=0.04) and neutropenia (OR 1.32, p=<0.001) for R/R AML. Since neutropenia was nearly universal among admitted pts with R/R AML, we repeated an MVA for readmissions in this group without including neutropenia as a covariate and found thrombocytopenia as a predictor for readmissions in R/R AML (OR 1.79, p=0.005). (Table 2) There was no significant association between the commonly used L.A.C.E. score and risk of readmissions among AML pts.
The 30-day mortality rate and inpatient mortality rate was 6% for unplanned induction readmissions, 11% for consolidation, and 18% for R/R AML. With the exception of induction, there was a higher incidence of 30-mortality for unplanned re-admissions during consolidation and R/R AML. The most common causes of death after readmission were infections with 57% for induction, 70% for consolidation, and 73% for R/R AML pts.
Conclusions Unplanned 30-day readmissions, most commonly due to infection, were common among patients with AML, with rates increasing with each sequential line of therapy. While the 30-day mortality and ICU admissions remain low for induction pts, those receiving consolidation or with R/R AML had significantly higher rates. We identified several factors, including hypoalbuminemia, thrombocytopenia, neutropenia and diabetes, as independent predictors for readmission. Implementing more frequent monitoring or trading increased length-of-stay for RaR in high-risk pts could help reduce mortality in AML.
Pemmaraju:Plexxikon: Research Funding; Incyte Corporation: Honoraria; Stemline Therapeutics: Honoraria, Research Funding; Cellectis: Research Funding; AbbVie: Honoraria, Research Funding; MustangBio: Honoraria; Celgene: Honoraria; Pacylex Pharmaceuticals: Consultancy; Affymetrix: Other: Grant Support, Research Funding; Samus Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; DAVA Oncology: Honoraria; Roche Diagnostics: Honoraria; SagerStrong Foundation: Other: Grant Support; Blueprint Medicines: Honoraria; LFB Biotechnologies: Honoraria; Novartis: Honoraria, Research Funding. DiNardo:Takeda: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria; ImmuneOnc: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Syros: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Novartis: Consultancy; Calithera: Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees. Daver:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Borthakur:Oncoceutics: Research Funding; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; BioTherix: Consultancy; Novartis: Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Abbvie: Research Funding; Jannsen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Research Funding; Polaris: Research Funding; Xbiotech USA: Research Funding; BioLine Rx: Consultancy; FTC Therapeutics: Consultancy; PTC Therapeutics: Research Funding; PTC Therapeutics: Consultancy; Argenx: Consultancy; Curio Science LLC: Consultancy; BMS: Research Funding. Cortes:Immunogen: Research Funding; Bristol-Myers Squibb: Research Funding; BiolineRx: Consultancy, Research Funding; Arog: Research Funding; Amphivena Therapeutics: Research Funding; Astellas: Research Funding; Telios: Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Merus: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sun Pharma: Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Research Funding. Ravandi:Amgen: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria. Kantarjian:Astex: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Research Funding; Novartis: Research Funding; Agios: Honoraria, Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding. Garcia-Manero:Novartis: Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Amphivena Therapeutics: Research Funding; H3 Biomedicine: Research Funding; Jazz Pharmaceuticals: Consultancy; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Onconova: Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Kadia:Ascentage: Research Funding; Astra Zeneca: Research Funding; Novartis: Honoraria; Amgen: Research Funding; Pulmotec: Research Funding; Astellas: Research Funding; Cyclacel: Research Funding; JAZZ: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Incyte: Research Funding; Celgene: Research Funding; Cellenkos: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal